 Accrediting agencies look for traceability processes that are compliant with evidence-based standards and guidelines. The trophon2 device with AcuTrace® RFID technology simplifies the creation of accurate digital records, easily obtained from your trophon2 device to support audit-readiness.
Accrediting agencies look for traceability processes that are compliant with evidence-based standards and guidelines. The trophon2 device with AcuTrace® RFID technology simplifies the creation of accurate digital records, easily obtained from your trophon2 device to support audit-readiness.
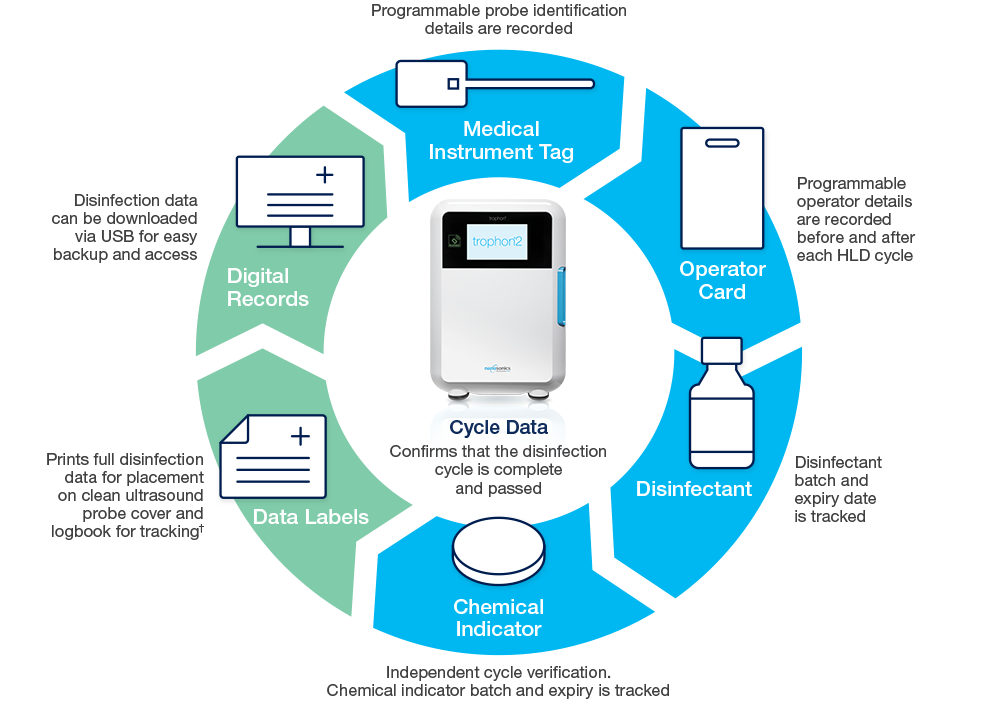
Automated Digital Traceability Across the Workflow
The trophon2 device increases user compliance and supports audit-readiness through automation, capturing data across the entire reprocessing workflow.
AcuTrace RFID technology records operator, probe and cycle data to capture and demonstrate user compliance.
AcuTrace-enabled consumables and accessories include:

Audit-Ready Compliance and Archiving
AcuTrace technology simplifies the creation of accurate digital records, accessible on your trophon2 device to support audit-readiness. AcuTrace technology electronically captures disinfection data in accordance with requirements in USA, CAN, ANZ, UK & EU.1-14
This data is easily accessible to suit a variety of user traceability methods:
- Printer label capturing AcuTrace inputs and disinfection results
- Download disinfection records to USB
- Optional AcuTrace® PLUS allowing API access, supporting digital disinfection data archiving*.

*Custom API middleware required to enable this capability. All connectivity, configuration and integration with customer IT systems is the responsibility of the customer.
The trophon® family includes trophon® EPR and trophon®2 which share the same core technology of 'sonically activated' hydrogen peroxide.
- AORN 2018. High-Level Disinfection. In: AORN Guidelines for periOperative Practice. Denver, CO.
- ANSI/AAMI ST58:2013 Chemical sterilization and high-level disinfection in health care facilities.
- CDC 2008 Guideline for Disinfection and Sterilization in Healthcare Facilities.
- AS/NZS 4815:2006 Office-based health care facilities - Reprocessing of reusable medical and surgical instruments and equipment, and maintenance of the associated environment.
- AS/NZS 4187:2014 Cleaning, disinfecting and sterilizing reusable medical and surgical instruments and equipment, and maintenance of associated environments in health care facilities.
- ACIPC/ASUM 2017. Guidelines for Reprocessing Ultrasound Transducers. Australasian Journal of Ultrasound in Medicine. 2017;20(1):30-40.
- Australian Commission on Safety and Quality in Health Care. National Safety and Quality Health Service Standards, second edition. November 2017.
- CAN/CSA-Z314-18. Canadian Medical Device Reprocessing. February 2018.
- Health Service Executive. Quality Improvement Division (2017). HSE Guidance for Decontamination of Semi‐critical Ultrasound Probes; Semi‐invasive and Non‐invasive Ultrasound Probes.
- Health Protection Scotland (2016). NHSScotland Guidance for Decontamination of Semi-Critical Ultrasound Probes; Semi-invasive and Non-invasive Ultrasound Probes.
- Welsh Health Technical Memorandum (2014). WHTM 01-06 - Decontamination of flexible endoscopes Part C: Operational management,
- Department of Health (UK) (2016). Health Technical Memorandum 01-06 Part C Operational management.
- Ministère des affaires sociales et de la santé (2016). INSTRUCTION N° DGOS/PF2/DGS/VSS1/2016/220 du 4 juillet 2016 relative à relative au traitement des endoscopes souples thermosensibles à canaux au sein des lieux de soins.
- Kommission für Krankenhaushygiene und Infektionsprävention and Bundesinstitut für Arzneimittel und Medizinprodukte. Anforderungen an die Hygiene bei der Aufbereitung von Medizinprodukten


